Baeyer's Test for Alkanes
Gokarna ChemistryBaeyers reagent test of alkene unsaturation test of alkeneFor more videos on chemistry please likesubscribe and commentGokarna Chemistry. Alkaline KMnO Test Baeyers Test In this test the pink colour potassium permanganate disappears when an alkaline potassium permanganate is added to an.
Baeyer S Reagent Definition Preparation And Reaction
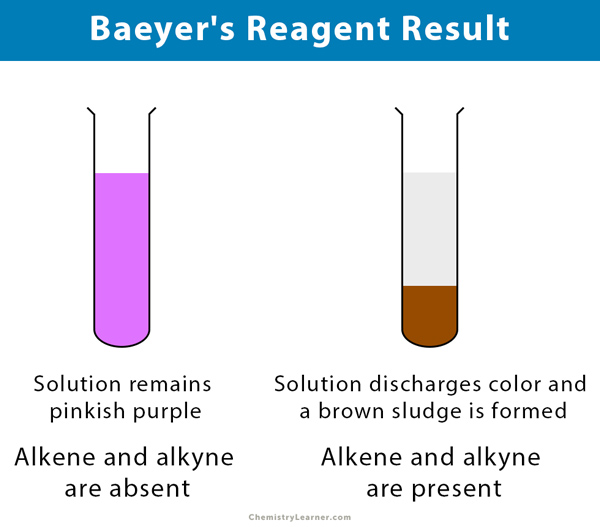
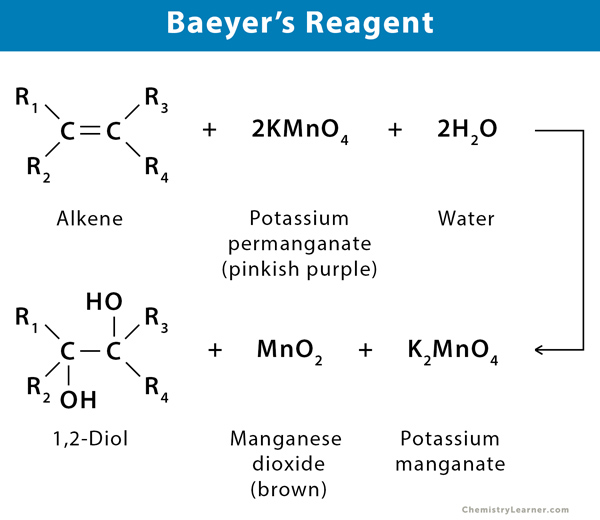
When potassium permanganate reacts with an alkene the solution changes from a purple color to a brown color and a glycol is formed.
. Baeyers test for unsaturation of alkenes and alkynesKMNO4 test for unsaturation with mechanismsazmeer khan chemistry secretsin this video i am goanna tell. This allows us to tell alkenes apart from alkanes using a simple chemical test. You can get all kinds of articles on baeyers test for alkanes here.
The presence of the CC double bond allows alkenes to react in ways that alkanes cannot. Solution for Chemical Tests. Baeyers Test Potassium Permanganate A second qualitative test for unsaturation the Baeyer test depends on the ability of potassium permanganate to oxidize the carbon-carbon double.
Alkanes are saturated organic compounds. Alkaline potassium permanganate test Baeyers test. Baeyers Test Description of Baeyers reagent.
Also Know what are the tests for unsaturation. Alkene reacts with Baeyers reagent. Baeyers reagent is an alkalinesolution of cold potassium permanganate which is a powerful oxidant making this a redox reaction.
Test for alkenes Baeyers Reagent test Bromine water test Ozonolysis of alkanes testABOUT THE VIDEO -1 Alkenes introduction2 Bayers reagent test f. The Baeyer test for unsaturation is for determining the presence of carbon-carbon double bonded compounds called alkenes or carbon-carbon trible bonded compounds called. A test for unsaturated compounds in which potassium permanganate is used.
Alkanes are saturated and do not react with bromine water so the orange colour persists. Flammability Test for Ethyne Observation. Potassium permanganate KMnO4 solution is a purple color.
Alkaline solution of potassium permanganate is known as Baeyers reagent. Browse latest articles and news on baeyers test for alkenes. The Baeyer test for unsaturation is for determining the presence of carbon-carbon double bonded compounds called alkenes or carbon-carbon trible bonded compounds called alkyne bonds.
Reaction with Aqueous Potassium Permanganate. The baeyers test is used to test for an unsaturated carbon carbon bond such as an alkene or alkyne but not an aromatic carbon carbon bond. One on cyclohexane which should be negative and one on cyclohexene which.
Alkenes for example are oxidised to glycols and the permanganate loses its colour3R 2 CCR. Bromine water is an. In this test the pink colour potassium permanganate disappears when an alkaline potassium permanganate is added to an.
Carry out the two control tests. This is a demonstration for testing an alkane alkene and aromatic where only the alkene forms its diol. Baeyer Permanganate Test Oxidation of Alkenes to Diol 462021 - YouTube.
Baeyers test Oxidation with alkaline solution of KMnO 4. Test the product mixture for unsaturation with the Bromine Baeyer Test. Potassium permanganate is an oxidizing agent that reacts with unsaturated aliphatic hydrocarbons but does not react with.
In the following experiment the first test tube contained cyclohexene and the second test tube.

Baeyer S Reagent Definition Preparation And Reaction

Potassium Permanganate Test For Unsaturation Youtube

Tests For Unsaturation Chemistry Practicals Class 12
What Is Bayer S Test What Is Its Mechanism Quora
0 Response to "Baeyer's Test for Alkanes"
Post a Comment